Artigo original
DORMANCY BREAK INDUCED BY GIBBERELLIC ACID AND IN VITRO GERMINATION OF SEEDS AND ZYGOTIC EMBRYOS OF PRUNUS CAMPANULATA MAXIM
Quebra de dormência induzida por ácido giberélico e germinação in vitro de sementes e embriões zigóticos de Prunus campanulata Maxim
https://doi.org/10.18593/evid.32526
Recebido em 22 de janeiro de 2023 | Aceito em 19 de março de 2023
Isabel Homczinski*
 Jonathan Matheus dos Santos†
Jonathan Matheus dos Santos† Daniela Sanson‡
Daniela Sanson‡
Alexandre Techy de Almeida Garrett§ Fabiana Schmidt Bandeira Peres¶
Fabiana Schmidt Bandeira Peres¶
Prunus campanulata is a species largely adopted for landscape composition in Brazil with limited seedling production by seed dormancy, lacking studies about in vitro propagation. This study aims to evaluate different P. campanulata seed pretreatments on germination, and the effect of GA3 on dormancy break and seedlings biometry. Seeds were processed in three treatments: complete seeds, seeds without endocarp, and isolated zygotic embryos incubated in a WPM medium containing different GA3 concentrations (0.0, 2.0, and 4.0 mg L-1). After 30 days of in vitro incubation were evaluated the germination percentage, mean germination time, percentage of normal seedlings, percentage of seedlings with leaves, and survival percentage were. Seedlings greater than 3.0 cm were selected for ex vitro adaptation, evaluating after 32 days the biometric variables: total length, aerial part length, root length, and the number of leaves. Complete seeds do not germinate, suggesting dormancy associated with the endocarp, whereas seeds without endocarp and isolated zygotic embryos showed the same germination rate, with no effect on GA3. The GA3 promoted greater seedling growth at concentrations between 1.89 mg L-1 for the total length of the isolated zygotic embryo and 2.24 mg L-1 for the length of the aerial part of seeds without endocarp. In conclusion, seed processing, i.e., removing the endocarp or isolating the zygotic embryo can overcome dormancy, improving germination and seedling production of P. campanulata in vitro.
Keywords: GA3. Japanese cherry tree. In vitro embryos. Biometry.
Prunus campanulata é uma espécie amplamente utilizada para composição de paisagens no Brasil com limitada produção de mudas por dormência de sementes, carecendo de estudos sobre propagação in vitro. Este trabalho tem como objetivo avaliar diferentes pré-tratamentos de sementes de P. campanulata na germinação e o efeito do GA3 na quebra de dormência e na biometria de plântulas. As sementes foram processadas em três tratamentos: sementes completas, sementes sem endocarpo e embriões zigóticos isolados incubados em meio WPM contendo diferentes concentrações de GA3 (0,0; 2,0 e 4,0 mg L-1). Após 30 dias de incubação in vitro foram avaliados o percentual de germinação, o tempo médio de germinação, o percentual de plântulas normais, o percentual de plântulas com folhas e o percentual de sobrevivência. Mudas maiores que 3,0 cm foram selecionadas para adaptação ex vitro, avaliando-se após 32 dias as variáveis biométricas: comprimento total, comprimento da parte aérea, comprimento da raiz e número de folhas. Sementes completas não germinam, sugerindo dormência associada ao endocarpo, enquanto sementes sem endocarpo e embriões zigóticos isolados apresentaram a mesma taxa de germinação, sem efeito sobre GA3. O GA3 promoveu maior crescimento de plântulas em concentrações entre 1,89 mg L-1 para o comprimento total do embrião zigótico isolado e 2,24 mg L-1 para o comprimento da parte aérea das sementes sem endocarpo. Em conclusão, o processamento de sementes, ou seja, a remoção do endocarpo ou o isolamento do embrião zigótico pode superar a dormência, melhorando a germinação e a produção de plântulas de P. campanulata in vitro.
Palavras-chave: GA3. Cerejeira japonesa. Embriões in vitro. Biometria.
@Autor correspondente: Doutora em Ciências Florestais (autora correspondente); Universidade Estadual do Centro Oeste (Unicentro). Rua Professora Maria Rosa Zanon, Engenheiro Gutierrez, Campus Irati, CEP: 84505-677, Irati, PR, Brasil; https://orcid.org/0000-0001-8178-2988; ihomczinski@gmail.com
Prunus campanulata Maxim. is a species of Rosaceae family (synonymous Cerasus campanulata (Maxim.) A.N. Vassiljeva and Prunus cerasoides var. campanulata (Maxim.) Koidz.)1 known as Japan cherry in Brazil, Taiwan cherry and bellflower cherry in other countries. The species occurs in China, Vietnam, and Japan, in forests and valleys at altitudes ranging from 100 to 1.300 m2. Japan cherry has great potential and value as an ornamental tree, due to its bellflower blossoms, besides a broad adaptation to different sites, since the species occurs in varied environments in its original distribution area3-4. In Japan, the species is a timber source being then introduced in several countries, such as the United Kingdom, United States, Australia, New Zealand4-5, and Brazil, where is used in parks, squares, and urban afforestation in the Southern region, flowering between June and July.
Although P. campanulata is considered an important species in the Southern, and its dispersion occurs mainly by seeds6, it is referred to the species the production of a reduced number of seeds for a short period7, associated with limited germination due to physiological dormancy of the embryo8-9. Dormancy is a phenome characterized by a viable seed that is unable to germinate, even under adequate environmental conditions10 being caused by endogenous factors, requiring pre-germinative treatments11. The main causes of tree seed dormancy are the presence of substances in the seed coat inducing embryo dormancy and limiting its growth. Moreover, dormancy can be an association of different factors, such as a physiological immature embryo with, a hard and impermeable seed coat, when the seed is unable to absorb water and oxygen for its development12-13.
Since dormancy set limitations to plant dispersion and seedling production, dormancy break techniques are needed, and in vitro vegetative propagation is an alternative for P. campanulata7. Among the advantages of this technique are embryogenesis control, the rescue of interspecific embryos, even when propagation limitations are present, haploid production, dormancy break, and production of aseptic seedlings can enable plant rescue and production. Other advantages of in vitro propagation are standardization and time reduction of seed germination, easy to identify and equalize limiting factors, such as nutritional and physiological embryo issues14-15.
Considering in vitro seed cultivation, zygotic embryos are commonly incubated in culture media containing salts, carbohydrates, and growth regulators16. Growth regulators are included in culture media to promote embryo germination and growth, gibberellins the most used, of which gibberellic acid (GA3) can promote seed dormancy break and germination, since, in addition to the seed coat limitations, the hormonal concentration and balance such between the gibberellic acid and abscisic acid influence dormancy break8,17. It is important to highlight further, that hormonal concentration is a critical factor since it can induce toxicity for seeds, inhibiting seed germination. In addition, can induce seedling anomalies by promoting enzyme activity, that degenerates cell walls11.
Presently, in vitro germination studies of P. campanulata are focused on tissue culture and mass propagation from axillary and adventitious buds via in vitro organogenesis6-7. In this way, information about the influence of gibberellic acid on dormancy break and germination of seed and zygotic embryos of P. campanulata in vitro, as well as on seedlings’ growth is still lacking. Thus, this study aims to evaluate different P. campanulata seed pretreatments on germination, and the effect of GA3 on dormancy break and seedlings biometry.
For this study, 180 fully mature fruits were collected in September, from four P. campanulata matrices, located on the Universidade Estadual do Centro-Oeste in the municipality of Irati, where the climate is classified as Cfb. The tests were carried out at the Silviculture Laboratory of Universidade Estadual do Centro-Oeste, Campus Irati, state of Parana (Unicentro).
The fruits were pulped and cleaned in running water to extract the seeds, being seeds separated into three processing treatments, each composed of matrices mix of 60 seeds: treatment 1 - complete seeds; treatment 2 - seeds without endocarp; and treatment 3 - isolated zygotic embryos. To remove the stony and woody integument, the seeds of treatments 2 and 3 were imbibed in filtered water for 24 hours, at room temperature. To remove the integument, pliers were used, cutting the ends of the seed.
For in-vitro incubation, processed seeds were disinfected in a laminar flow chamber, immersing seed in 70% alcohol (v/v) for one minute followed by immersion in 2.5% sodium hypochlorite (v/v) added with three drops of surfactant Tween 20® for each 100 mL of solution, for 15 minutes, under constant agitation. Then, the seeds were washed three times in sterile deionized water18. After disinfestation, isolation of the zygotic embryos from the seeds of Treatment 3 was carried out in a sterile condition, maintaining part of the integument. This procedure was performed with tweezers and a scalpel, with the aid of a stereoscopic microscope with a 40x magnification.
Seeds were incubated in nutritive WPM (Wood Plant Medium) medium19. To the WPM medium was added myo inositol (100 mg L-1); sucrose (30 g L-1), and different concentrations of GA3, comprising three treatments: 0.0, 2.0, and 4.0 mg L-1. According to Faria et al.20 and Pio et al.21, sucrose is a source of carbohydrates for the embryo and favors the formation of the root in the seedling, that is, it favors germination.
After preparation, the medium pH was adjusted to 5.7 ± 0.1, adding to the medium melted agar at a concentration of 6 g.L-1. The different GA3 treatments were poured into test tubes (150 x 25 mm) sealed with a cap, each with 10 ml of medium, and sterilized in an autoclave at 120 ºC (1.5 atm) for 20 minutes. The seeds and isolated zygotic embryos were deposited in the test tubes, and incubated in a growth room, with a photoperiod of 16 hours of light and 8 hours of darkness, a temperature of 25 ± 2 ºC, and irradiance of 27 μmol m-2 s-1.
2.4 STATISTICAL DESIGN AND ANALYSIS
The experiment was in a completely randomized design, in a factorial scheme (3 seed processing treatments x 3 GA3 concentrations) with four replications and five seeds per replication, with each seed and isolated zygotic embryo individually incubated in the test tubes. The experiment totaled 180 seeds, 15 for each treatment. After 30 days of in vitro incubation, germination was evaluated by the following variables: germination percentage (G) (Equation 1), mean germination time (MGT) (Equation 2)22, percentage of normal seedlings (NS), percentage of seedlings with leaves (SL) and survival percentage (S).
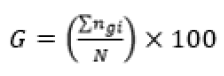 (Equation 1)
(Equation 1)
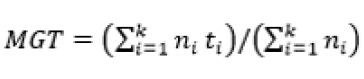 (Equation 2)
(Equation 2)
Where: N – number of incubated seeds; – total number of germinated seeds; t – average germination time; – number of seeds that germinate in time ; – time between the beginning of the experiment and the i-th (day or hour) observation; k – last day of observation.
After the evaluation of in vitro germination, seedlings that emerged and reached a length greater than 3 cm were selected for ex-vitro adaptation. These were transplanted into glass pots containing substrate based on bio-stabilized pine bark, previously sterilized in an autoclave at 120 ºC (1.5 atm) for 40 minutes, which were sealed with two layers of plastic film in the growth room.
After 32 days of adaptation, seedlings biometric evaluation was carried out, evaluating: total length (TL, cm), aerial part length (AL, cm), root length (RL, cm), and leaves number (LN, unit). Seedlings, obtained from in vitro germination, were evaluated in an unbalanced completely randomized design, with a variable number of repetitions per treatment, in a factorial scheme (2x3), in which Factor 1 consisted of seeds without endocarp and isolated zygotic embryos and the Factor 2 constituted by the three concentrations of GA3. Each seedling was considered a repeat in a total of 68 seedlings.
The data from the evaluations of in vitro germination and biometry of P. campanulata seedlings were submitted to the Levene and Bartlett test (p-value ) respectively. The homogeneous variances had the effects of the treatments tested using the ANOVA F test, while those that showed heterogeneity had the original values transformed into (germination and survival percentages), (percentage of normal seedlings) and (percentage of seedlings with leaves), where: arc sen is the arcosene, x is the variable of interest and ln is the Neperian logarithm, for further analysis by the same means test.
For statistical analysis, software R23 was used with the “ExpDes.pt” package24 and the “car” package25. The treatment means were compared using the Tukey mean test, considering a significance of 5%.
To represent the relationship between GA3 concentrations, in mg L-1, and biometric variables, quadratic regression equations were adjusted. The trend curves were presented for the four variables evaluated, with the best-adjusted equation, presenting the ideal maximum point, coefficient of determination (R2), and the standard error in percentage (Syx%).
Germination was recorded from the third day after seed incubation, with no germination observed for complete seeds. The isolated zygotic embryos (ZE) promoted better germination (63.33%), percentage of normal seedlings (79%), percentage of seedlings with leaves (97%), and survival (61.67%) rates, except for the mean germination time, which was lower for seeds without endocarp (WE) (6 days) (Table 1).
After germination evaluations, no statistical differences were observed for the evaluated variables (G, MGT, NS, SL, and S) among GA3 concentrations and for the interaction of seed processing treatments (seeds without endocarp and isolated embryos) x GA3 (Table 2).
Regarding seedlings biometry, only seedlings showing normal development were evaluated. Seedlings from seeds without endocarp had the greatest mean for total length (9.40 cm) and aerial part length (5.55 cm). Root length (4.23 cm) and the number of leaves (3.87 units) were promoted on seedlings from the isolated embryos. However, maximum values were observed for seedlings from seeds with seed coats (removed endocarp) (Table 3).
For the seed factor, there was no statistically significant difference for the evaluated biometric variables. However, for the GA3 factor (mg L-1) there was a statistically significant difference in the variables among the concentrations, with the concentration of 2 mg L-1 providing greater seedling development. The seed processing treatments x GA3 interaction was also statistically significant, with greater development of seedlings from isolated zygotic embryos at a concentration of 2 mg L-1 GA3, except for the root length variable (Table 4).
Table 1 – Descriptive statistics of the variables evaluated after in vitro incubation of isolated zygotic embryos and seeds without endocarp of Prunus campanulata in different GA3 concentrations
|
Statistics |
Isolated zygotic embryos |
Seeds without endocarp |
|||||||||
|
G |
MGT |
NS |
SL |
S |
G |
MGT |
NS |
SL |
S |
||
|
Minimum |
20.00 |
4 |
33.33 |
66.67 |
20.00 |
20.00 |
3 |
0 |
0 |
20.00 |
|
|
Mean |
63.33 |
8 |
79.17 |
97.22 |
61.67 |
51.67 |
6 |
67.36 |
91.67 |
51.67 |
|
|
Maximum |
100 |
16 |
100 |
100 |
100 |
100 |
8 |
100 |
100 |
100.00 |
|
|
Standard Error |
6.44 |
1 |
7.07 |
2.78 |
6.26 |
7.57 |
0 |
11.44 |
8.33 |
7.57 |
|
|
Variation Coefficient (%) |
35.20 |
48.02 |
30.93 |
9.90 |
35.14 |
50.76 |
23.20 |
58.85 |
31.49 |
50.76 |
|
G: percentage of germination; MGT: mean germination time; NS: percentage of normal seedlings; SL: percentage of seedlings with leaves; S: survival percentage.
Table 2 – Average germination percentage (G), average germination time (MGT), normal seedlings percentage (NS), seedlings with leaves percentage (SL), and survival percentage (S) of Prunus campanulata seeds without endocarp (WE) and isolated zygotic embryos (ZE) in the presence of different concentration of GA3 in vitro
|
GA3 |
G |
MGT |
NS |
SL |
S |
|||||||||
|
WE |
ZE |
WE |
ZE |
WE |
ZE |
WE |
ZE |
WE |
ZE |
|||||
|
0 mg L-1 |
60 |
75 |
6.16 |
7.59 |
100 |
93.75 |
100 |
100 |
60 |
75 |
||||
|
2 mg L-1 |
50 |
65 |
5.77 |
6.04 |
39.58 |
64.58 |
75 |
91.67 |
50 |
60 |
||||
|
4 mg L-1 |
45 |
50 |
6.58 |
10.92 |
62.5 |
79.17 |
100 |
100 |
45 |
50 |
||||
|
Mean |
51.67 |
63.33 |
6.0 |
8.0 |
67.33 |
79.17 |
91.67 |
97.25 |
51.67 |
61.67 |
||||
Table 3 – Descriptive statistics of the biometric variables analyzed during the in vitro incubation of Prunus campanulata seedlings in different concentrations of GA3
|
Statistics |
Isolated zygotic embryos |
Seeds without endocarp |
|||||||
|
TL (cm) |
LA (cm) |
RL (cm) |
LN |
TL (cm) |
LA (cm) |
RL (cm) |
LN |
||
|
Minimum |
2.40 |
0.90 |
0.60 |
1.00 |
0.90 |
0.60 |
2.00 |
0.30 |
|
|
Mean |
9.06 |
4.84 |
4.23 |
3.87 |
9.40 |
5.55 |
4.19 |
3.85 |
|
|
Maximum |
15.20 |
9.90 |
8.50 |
10.00 |
21.70 |
13.40 |
14.00 |
9.20 |
|
|
Standard Error |
0.68 |
0.40 |
0.36 |
0.42 |
0.88 |
0.54 |
0.38 |
0.37 |
|
|
Variation Coefficient (%) |
41.55 |
46.13 |
47.98 |
60.67 |
56.68 |
59.39 |
54.50 |
59.04 |
|
TL: Total length; LA: length of the aerial part; RL: root length; LN: leaves number.
Table 4 – Summary of the analysis of variance of the biometric variables analyzed during the in vitro incubation of Prunus campanulata seedlings in different concentrations of GA3
|
Variation |
GL |
Mean squares |
|||
|
TL (cm) |
LA (cm) |
RL (cm) |
LN (unit) |
||
|
Seeds processing |
1 |
1.90 ns |
8.64ns |
2.43 ns |
0.25 ns |
|
GA3 |
2 |
262.99** |
117.87** |
29.10** |
2,19** |
|
Seeds x GA3 |
2 |
60.73* |
22.56** |
9.32ns |
0.99** |
|
Residue |
62 |
12.89 |
4.19 |
3.74 |
0,19 |
|
Total |
67 |
- |
- |
- |
- |
|
Mean |
5.23 |
4.02 |
9.24 |
4,04 |
|
|
Variation Coefficient (%) |
38.84 |
38.84 |
48.14 |
47.13 |
|
|
Bartlett test (p-value) |
0.79 ns |
0.79 ns |
0.70 ns |
0.18 ns |
|
TL: Total length; LA: length of the aerial part; RL: root length; LN: leaves number. (*) Significant F test at 5% probability of error; (**) significant at the 1% probability of error level; (ns) not significant.
All biometric variables were represented by a polynomial second-degree equation (Figure 1 a-d). The same response was not observed for the number of leaves for seedlings from isolated zygotic embryos, represented by a linear equation explaining 99% of the data dispersion (Figure 1d). The root length showed no interaction between the factors, being evaluated only concerning GA3 concentrations. According to the regression analysis the ideal GA3 concentrations were: for total length 1.89 mg L-1 for isolated zygotic embryo, and 2.22 mg L-1 for seeds without endocarp; for length of aerial part 1.91 mg L-1 for isolated zygotic embryo, and 2.24 mg L-1 for seeds without endocarp; for root length 1.87 mg L-1 for isolated zygotic embryo, and 2.16 mg L-1 for seeds without endocarp; for leaf number 9.55 mg L-1 for isolated zygotic embryo, and 2.03 mg L-1 for seeds without endocarp.
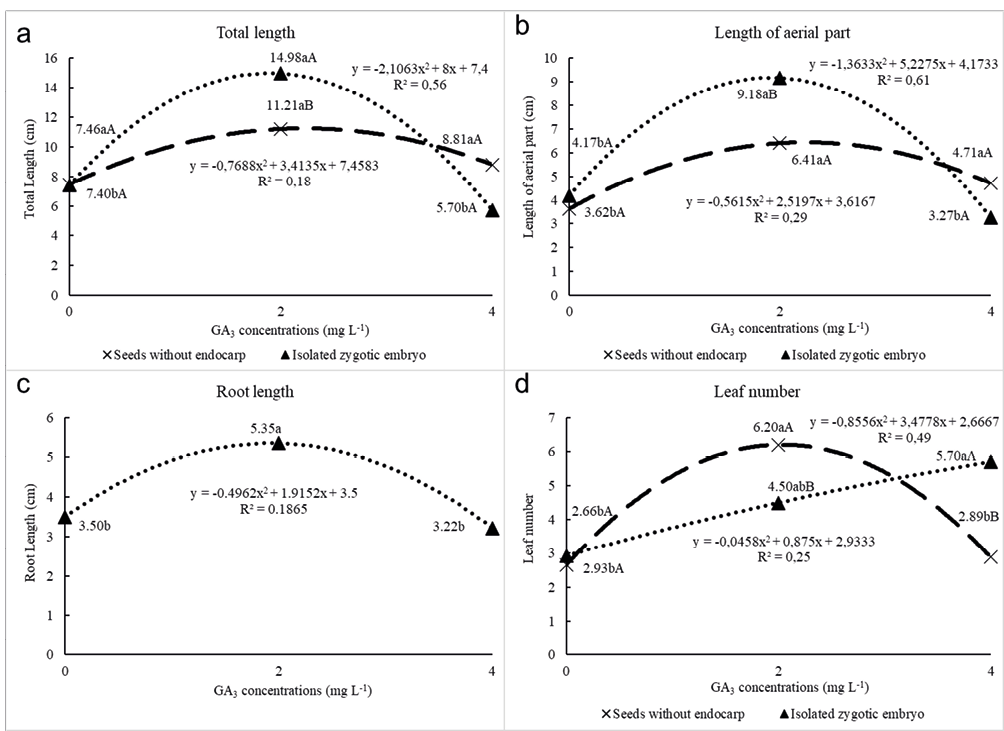
Figure 1 – Adjusted regression equations and determination coefficients (R²) for the relationship between GA3 concentrations (mg L-1) for total length (cm) (A), length of the aerial part (cm) (B), root length (cm) (C), and leaf number (unit) (D) in vitro cultivation of Prunus campanulata seedlings. Means followed by vertical capital letters refer to the interaction of seed processing treatments within the same ga3 concentration; lowercase letters horizontally refer to the interaction of GA3 concentrations within the same seed processing treatment. Equal letters do not differ statistically from each other, to 5% probability of error by Tukey Test.
Seedlings from isolated zygotic embryos such as the seedlings at a concentration of 2 mg L-1 GA3 showed better development (Figure 2e). It was also verified that the seedlings cultivated in the medium containing GA3 at a concentration of 4 mg L-1 (Figures 2c and 2f) had a shorter length when compared to the others, showing curled leaves and aerial part deformation, characterizing toxicity.

Figure 2 – Seedlings of Prunus campanulata at 32 days of in vitro incubation. Seedling from seeds without endocarp: (A) 0 mg.L-1 GA3; (B) 2 mg.L-1 GA3; (C) 4 mg.L-1 de GA3; Seedling from isolated zygotic embryos: (D) 0 mg.L-1 de GA3; (E) 2 mg.L-1 de GA3; (F) 4 mg.L-1 de GA3. Scale bars = 10 mm
Since no germination was observed for complete seeds, the results herein suggested the presence of mechanical dormancy due to the stony and woody endocarp of P. campanulata. This characteristic is corroborated by the seed processing treatments, i.e. seeds without tegument and isolated zygotic embryos, as germination was greater than 50% for both treatments, although no statistical differences were observed between these treatments.
The dormancy of P. campanulata seeds is assigned to endocarp influences associated with hormonal balance8. However, the presence of an endocarp does not seem to be limiting26, as this structure is permeable when mature8. The same is valid for P. yedoensis, in which germination is limited by hormonal balance and not by physical obstruction caused by the seed coat9. Moreover, according to Fowler and Bianchett27 mechanical dormant seeds do not germinate even under favorable conditions, demanding dormancy break treatments, such as scarification, stratification, water immersion, or complete endocarp and tegument removal. In this way, the authors also highlighted that when the zygotic embryo is isolated the germination is observed, as well as observed for P. campanulata in this study.
Regarding the regulation of hormonal balance in seed dormancy, Lee et al.10 observed protein expression variations in seeds of P. campanulata after stratification (applying high and low temperatures), and as the protein expression changed the hormonal balance changed as well, causing abscisic acid (ABA) reduction up to 12 times and an increase of gibberellins in the seeds, influencing dormancy regulation17. Thus, hormonal changes, as well as exogenous gibberellin application, can remove the chemical barrier that limits radicle development, similarly to the stratification technique8. Besides that, Chen et al.8 identified that ABA contents are greater in the endocarp in comparison to the seed coat and the embryo, moreover, when the seed endocarp was removed, the seed dormancy was removed. Finally, dormancy can be genetically controlled in the seeds, so this character can be explored in breeding programs, such as for P. persica (L.) Batsch17.
Dormancy on P. campanulata can be associated with combined mechanical resistance and hormonal balance in the tegument since Chen et al.8 observed that when the endocarp is removed, the dormancy is suppressed. On the other hand, it is important to highlight that seed imbibition in water, such as in this study for seeds with seed coat and isolated zygotic embryos, can contribute to germination, once seed wash ensures chemical dormancy break on seed layers27.
In addition, the results of this study also suggest dormancy caused by the tegument, since the application of exogenous gibberellin does not enhance germination, even though gibberellins act in seed development28 and activates DNA of aleurone cells for α-amylase production29. Moreover, it was observed an increase in seed germination after seed processing treatments. The use of sodium hypochlorite in the disinfection process may be related to reductions in seed germination, since the salt changes gibberellin and abscisic acid ratio reducing active gibberellin levels, impairing seed germination30, which is reduced by GA3 treatment31.
According to Chen et al.8 P. campanulata dormancy is not physiologic, as endocarp remotion promoted germination rate and time reduction. In contrast, in this study seeds without endocarp had the same germination rate as isolated zygotic embryos8. Similar results were observed for zygotic embryos of Byrsonima cidoniifolia A. Juss. incubated in vitro, being low germination rate is attributed to limitations induced by the endocarp and dormancy, thus the use of zygotic embryos in vitro can contribute to reducing germination time and promote the production of viable seedlings without contaminants32.
Considering the techniques to promote dormancy break, although stratification increases P. campanulata germination rate, this technique demands a long period, up to 14 weeks10 or even 18 weeks8. In this context, removing the endocarp and exogenous application of GA3 can be viable alternatives to obtain seedlings since these techniques can improve germination rates9. Furthermore, when P. campanulata endocarp is removed or the embryo is isolated, it allows a germination time ranging from six to eight days, similar to seven to 13 days for stratified seeds as observed by Chen et al.8, evidencing the advantage of the seed processing treatments used in this study.
The germination percentage in this study was lower than observed by Lee et al.10 ranging from 72% (12 weeks) to 98% (14 weeks) for stratified seeds, and by Chen et al.8 of 100% and 25% for isolated zygotic embryos and seeds without endocarp, respectively, after 21 days. Despite that, the simple seed imbibition and endocarp remotion in addition to in vitro incubation substantially improved germination rate and time, as germination was observed three days after incubation originating normal seedlings appropriate for ex vitro adaptation in less than 30 days. Germination was not induced by the different GA3 concentrations tested, contrasting the effects observed by Grisez et al.26, reporting that gibberellin is effective in promoting germination after endocarp remotion. Similarly, Chen et al.8 highlighted that the phytoregulator is partially effective on unstratified intact seeds, however, when the endocarp is removed, GA3 application can promote germination. On the other hand, Kim9 reported the benefic effects of GA3 on the germination of intact P. yedoensis seeds, reaching a rate of 71%, suggesting that dormancy is associated with the hormonal ratio in the seeds. Finally, exogenous gibberellin application can contribute to the germination uniformity of Prunus species.
Development impairment by high GA3 concentrations was reported herein for zygotic embryos of other woody species incubated in vitro33-34. In this context, Mendes et al.11 highlighted that high concentrations of gibberellins can result in toxicity limiting seedling development. High concentrations can lead to anomalies as enzyme activity increases in such situations, causing cell wall degradation, corroborating the results observed in this study. In this research, although GA3 did not promote significant effects on germination, the phytoregulator induced biometric changes, improving aerial part and root growth and leaf formation. However, among concentrations was observed growth impairment when the gibberellin concentration was 4 mg L-1, in comparison to the seeds incubated without GA3 (Figure 2), suggesting that high concentrations cause the plant to be known as reported by Mendes et al.11
In conclusion, the germination only of seeds without endocarp and isolated zygotic embryos confirmed the presence of mechanical dormancy of P. campanulata, which was not influenced by the GA3 concentrations tested. On the other hand, endocarp remotion in addition to in vitro incubation promoted a greater germination rate, whereas GA3 promoted greater seedling growth at concentrations between 1.89 mg L-1 for a total length of isolated zygotic embryo and 2.24 mg L-1 for the length of aerial part of seeds without endocarp.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
1. Huang KF, Wen CH, Wang CT, Chu FH. Transcriptome and flower genes analysis of Prunus campanulata Maxim. The Journal of Horticultural Science and Biotechnology [Internet]. 2019 Jul 22;95(1):44-52. Available from: http://dx.doi.org/10.1080/14620316.2019.1641163
2. Cheng T, Weng Y, Yang L, Lu L, Hao Z, Shi J, et al. The chloroplast genome of Cerasus campanulata (Maxim.) A.N. Vassiljeva. Mitochondrial DNA Part B [Internet]. 2018 Jan 2;3(1):222-4. Available from: http://dx.doi.org/10.1080/23802359.2018.1437799
3. Chen Z, Shi J, et al. Research advance, prospect, and breeding strategy of Cerasus campanulata maxim. Journal of Nanjing Forestry University (Natural Sciences Edition). 2006;30(1):115-8.
4. Yi XG, Chen J, Zhu H, Li YF, Li XX, Li M, et al. Phylogeography and the population genetic structure of flowering cherry Cerasus serrulata (Rosaceae) in subtropical and temperate China. Ecology and Evolution [Internet]. 2020 Sep 13;10(20):11262-76. Available from: http://dx.doi.org/10.1002/ece3.6765
5. Cottrell V. Prunus campanulata (Taiwan cherry). CABI Compendium [Internet]. 2022 Jan 7; CABI Compendium. Available from: http://dx.doi.org/10.1079/cabicompendium.44268
6. Chen B, Li J, Zhang J, Wu Z, Fan H, Li Q, et al. Optimizing the rapid technique for propagation of Cerasus campanulata by tissue culture. Pak J Bot. 2016;48(1):305-9.
7. Zhang Y, Rong J, Fu Y, Chen L, Chen L, Zheng Y, et al. Tissue culture and plant regeneration of Prunus ampanulate Maxim. JAPS, Journal of Animal and Plant Sciences. 2015;25(Suppl. 1):146-51.
8. Chen SY, Chien CT, Chung JD, Yang YS, Kuo SR. Dormancy-break and germination in seeds of Prunus campanulata (Rosaceae): role of covering layers and changes in concentration of abscisic acid and gibberellins. Seed Science Research [Internet]. 2007 Mar;17(1):21-32. Available from: http://dx.doi.org/10.1017/S0960258507383190
9. Kim DH. Practical methods for rapid seed germination from seed coat-imposed dormancy of Prunus yedoensis. Scientia Horticulturae [Internet]. 2019 Jan;243:451-6. Available from: http://dx.doi.org/10.1016/j.scienta.2018.08.039
10. Lee CS, Chien CT, Lin CH, Chiu YY, Yang YS. Protein changes between dormant and dormancy-broken seeds of Prunus campanulata Maxim. PROTEOMICS [Internet]. 2006 Jul;6(14):4147-54. Available from: http://dx.doi.org/10.1002/pmic.200500118
11. Mendes RG, Silva Bonetti LL da, Gastl Filho J, Menezes DP de, Santi SL de, Rezende AS, et al. Germinação e vigor de sementes de araticum-cagão influenciados por GA3 em diferentes substratos. Brazilian Journal of Animal and Environmental Research. 2019;2(1):632-45.
12. Pacheco MV, Matos VP. Método para superação de dormência tegumentar em sementes de Apeiba tibourbou aubl. Revista Brasileira de Ciências Agrárias – Brazilian Journal of Agricultural Sciences [Internet]. 2009 Mar 3;4(1):62-6. Available from: http://dx.doi.org/10.5039/agraria.v4i1a10
13. Guedes RS, Alves EU, Viana JS, Gonçalves EP, Santos S do RN dos, Costa EG da. Tratamentos pré-germinativos e temperaturas para a germinação de sementes de Apeiba tibourbou Aubl. Revista Brasileira de Sementes [Internet]. 2011;33(1):131-40. Available from: http://dx.doi.org/10.1590/S0101-31222011000100015
14. Kumar N, Reddy MP. In vitro plant propagation: A review. Journal of Forest and Environmental Science [Internet]. 2011 Aug 31;27(2):61-72. Available from: https://doi.org/10.7747/JFS.2011.27.2.1
15. Lencina KH, Bisognin DA, Kielse P, Pimentel N, Fleig FD. Estabelecimento e crescimento in vitro de plantas de grápia. Ciência Rural [Internet]. 2014 Jun;44(6):1025-30. Available from: http://dx.doi.org/10.1590/S0103-84782014000600012
16. Jesus AMS, Villa F, Lara AC da C, Pasqual M. Avaliação do efeito das concentrações de sacarose e dos estádios de desenvolvimento do fruto no cultivo in vitro de embriões de frutos de cafeeiro. Revista Ceres [Internet]. 2011 Dec;58(6):679-84. Available from: http://dx.doi.org/10.1590/S0034-737X2011000600001
17. Kanjana W, Suzuki T, Ishii K, Kozaki T, Iigo M, Yamane K. Transcriptome analysis of seed dormancy after rinsing and chilling in ornamental peaches (Prunus persica (L.) Batsch). BMC Genomics [Internet]. 2016 Aug 8;17(1). Available from: http://dx.doi.org/10.1186/s12864-016-2973-y
18. Fermino Junior P, Nagao EO, Scherwinski-Pereira JE, et al. In vitro establishment, germination, and multiplication of teak (Tectona grandis Lf) from genotypes of southwestern amazon. Scientia Forestalis. 2009;37(84):427-35.
19. Lloyd BH G. and McCown. Commercially-feasible micropropagation of mountain laurel, kalmia latifolia, by use of shoot-tip culture. Combined Proceedings-International Plant Propagator’s Society. 1980;30:421-7.
20. Faria GA, Costa MAP de C, Junghans TG, Ledo CA da S, Souza A da S. Efeito da sacarose e sorbitol na conservação in vitro de Passiflora giberti N. E. brown. Revista Brasileira de Fruticultura [Internet]. 2006 Aug;28(2):267-70. Available from: http://dx.doi.org/10.1590/S0100-29452006000200025
21. Pio R, Ramos JD, Pio LAS, Mendonça V, Silva ABD, Pasqual M. DE CITROS Tangerina sunki x trifoliata english 63-256 COM O USO. Ciênc agrotec. 2002.
22. Labouriau LG. A germinação das sementes. Secretaria Geral da Organização dos Estados Americanos, Washington; 1983.
23. R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available from: https://www.R-project.org/
24. Ferreira EB, Cavalcanti PP, Nogueira DA. ExpDes.pt: Pacote experimental designs (portugues). 2021. Available from: https://CRAN.R-project.org/package=ExpDes.pt
25. Fox J, Weisberg S. An r companion to applied regression. 2019. Available from: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
26. Grisez TJ, Barbour JR, Karrfalt P. Prunus l. Seeds of woody plants in the United States Agriculture Handbook. 1974;450:658-73.
27. Fowler JAP, Bianchetti A. Dormência em sementes florestais. Colombo: Embrapa Florestas; 2000.
28. Vignati E, Lipska M, Dunwell JM, Caccamo M, Simkin AJ. Options for the generation of seedless cherry, the ultimate snacking product. Planta [Internet]. 2022 Sep 28;256(5). Available from: http://dx.doi.org/10.1007/s00425-022-04005-y
29. Kingsley Mbi T, Godswill Ntsefong N, Eugene Lenzemo T. Seed dormancy: Induction, maintenance and seed technology approaches to break dormancy. In IntechOpen; 2022. Available from: http://dx.doi.org/10.5772/intechopen.106153
30. Shu K, Qi Y, Chen F, Meng Y, Luo X, Shuai H, et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Frontiers in Plant Science [Internet]. 2017 Aug 10;8. Available from: http://dx.doi.org/10.3389/fpls.2017.01372
31. Chauhan A, AbuAmarah BA, Kumar A, Verma JS, Ghramh HA, Khan KA, et al. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi Journal of Biological Sciences [Internet]. 2019 Sep;26(6):1298-304. Available from: http://dx.doi.org/10.1016/j.sjbs.2019.04.014
32. Martendal CDO, Bernardino MM, Pereira FD, Silva FG, De Menezes CCE, Monteiro Hara ACB de A. In vitro cultivation of zygotic embryos from murici (Byrsonima cydoniifolia a. Juss.): Establishment, disinfection, and germination. Acta Scientiarum Agronomy [Internet]. 2013 Mar 26;35(2). Available from: http://dx.doi.org/10.4025/actasciagron.v35i2.15402
33. Soares FP, Paiva R, Stein VC, Nery FC, Nogueira RC, Oliveira LM de. Efeito de meios de cultura, concentrações de GA3 e pH sobre a germinação in vitro de mangabeira (Hancornia speciosa Gomes). Ciência e Agrotecnologia [Internet]. 2009;33(spe):1847-52. Available from: http://dx.doi.org/10.1590/S1413-70542009000700025
34. Muniandi SKM, Hossain MdA, Abdullah MohdP, Ab Shukor NA. Gibberellic acid (GA3) affects growth and development of some selected kenaf (Hibiscus cannabinus L.) cultivars. Industrial Crops and Products [Internet]. 2018 Aug;118:180-7. Available from: http://dx.doi.org/10.1016/j.indcrop.2018.03.036
* Doutora em Ciências Florestais (autora correspondente).
† Mestrado em Ciências Florestais; Universidade Estadual do Centro Oeste (Unicentro).
‡ Doutoranda em Ciências Florestais; Universidade Estadual do Centro Oeste (Unicentro).
§ Pós-Doutor Ciências Florestais; Universidade Estadual do Centro Oeste (Unicentro).
¶ Doutora em Ciência Florestal; Universidade Estadual do Centro Oeste (Unicentro).